Isomer Definition
Isomers are two molecules with the same molecular formula but differ structurally. Therefore, isomers contain the same number of atoms for each element, but the atomic arrangement differs. Despite having the same molecular formula, the physical properties of each molecule may differ, particularly if the functional groups associated with each molecule are different.
Isomerization is the process by which one molecule is converted into another molecule with the identical atoms. This may occur spontaneously or a reaction may be required to achieve this effect.
Types of Isomers
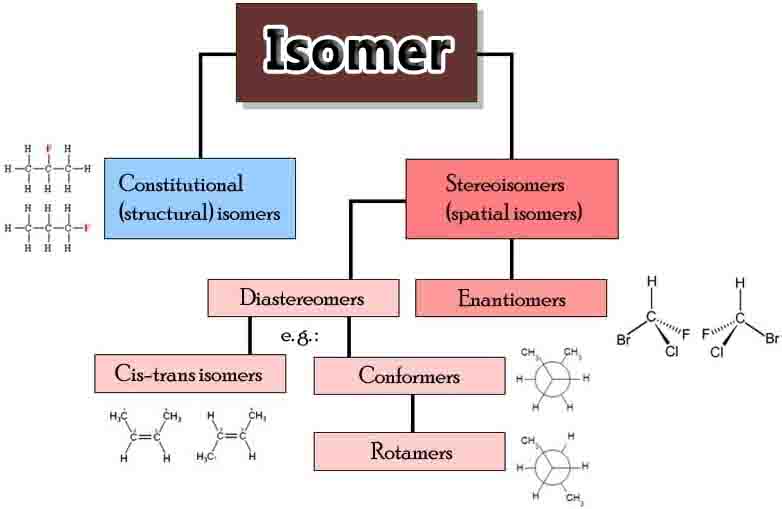
There are two main types of isomers, structural isomers and stereoisomers (illustrated below).
Structural isomers
Structural isomers differ with regards to the specific attachment of atoms and functional groups. Thus, depending on the specific isomers, they may not be classified under the same functional group and they will have different IUPAC names.
Types of structural isomers include chain isomers (e.g., hydrocarbon chains exhibiting different branching patterns); position isomers, which differ based on the positioning of a functional group on the chain; functional group isomers, in which a functional group is further divided into different functional groups; and skeletal isomers, which exhibit different carbon chains.
Another type of structural isomer is a tautomer. Tautomers spontaneously interconvert between two structural isomers, and exhibit different properties depending on the particular isoform. Occasionally, tautomer conversion can be so rapid that the isolation of both is not possible.
Stereoisomers
Stereoisomers refer to isomers which share an identical bond structure but differ with regards to the geometric position of the functional groups and atoms. Types of stereoisomers consist of enantiomers, diastereomers, and conformational isomers.
Enantiomers are mirror-images which contain chiral centers and are not superimposable. Diastereomers are not mirror-images, which may or not contain chiral centers. Conformational isomers exhibit different rotations around single bonds.
Example of an Isomer
There are several examples of isomers, described as follows:
Methoxyethane and Propanol
The chemical structure, C3H8O exists as several isomers of propanol, as well as the isomer methoxyethane. The two propanol isomers consist of propan-1-ol and propan-2-ol (also known as isopropyl alcohol), which are distinguished by the placement of an oxygen atom either on the terminal carbon atom or the central carbon atom, respectively.
Methoxyethane is also an isomer of C3H8O, but is an ether due to the placement of an oxygen atom in the center of the molecule, rather than connected to a single carbon atom.
Methylacetylene and Allene
Methylacetylene and allene are an example of C3H4 isomers, which differ based on the type of bonding exhibited by the molecules. Methylacetylene has one triple bond and one single bond between the carbon atoms and allene exhibits two double bonds between the carbons.
Fulminate and Cyanate
Fulminate and cyanate are an example of isomers of CNO. Fulminate exhibits an arrangement in which the N is bound to both the C and O atoms, whereas in cyanate, both the O and N are bound to the central C atom.
Glucose and Fructose
Glucose and fructose are an example of C6H12O6 isomers, which differ based on the position of a double bonded O atom. In glucose, the O is situated on the first C, whereas it is located on the second C in fructose (the structures of each are shown below).
Pharmaceutical Examples
Isomers are extremely important in the development of pharmaceuticals, as typically, only one isomer of a particular molecule will exert the desired effect. For example, only one isomer for ibuprofen will bind to the necessary target in the human body and induce pain relief. Similarly, cisplatin is an effective anticancer drug, whereas its isomer, transplatin, exhibits no anti-cancer benefits at all.
One of the most famous examples is that of thalidomide (pictured below). While one isomer of thalidomide is a potent morning sickness suppressant, it was delivered at a 50:50 ratio with its stereoisomer, which was responsible for the birth defects observed in the children born in the 1950’s from mothers who took the drug.
Enzymatic Reactions
One of the most important functions of isomers in the human body is that of enzymatic reactions. The orientation, functional groups, bond lengths, and overall 3D structure of a molecule impacts its ability to bind to enzymes. Enzymes typically recognize a specific molecular shape similar to a lock and key.
Therefore, isomers which have a different physical shape will not be able to bind to a particular enzyme, despite having the same molecular formula. An example of this is the enzyme triose-phosphate isomerase, which is involved in glycolysis by catalyzing the interconversion of dihydroxyacetone and (R)-glyceraldehyde phosphate; however, the isomer (S)-glyceraldehyde does not achieve the same reaction as it does not fit into the triose-phosphate enzyme.
Enzymes that function to convert molecules into their isomers (e.g., triose-phosphate isomerase described above) are called isomerases.