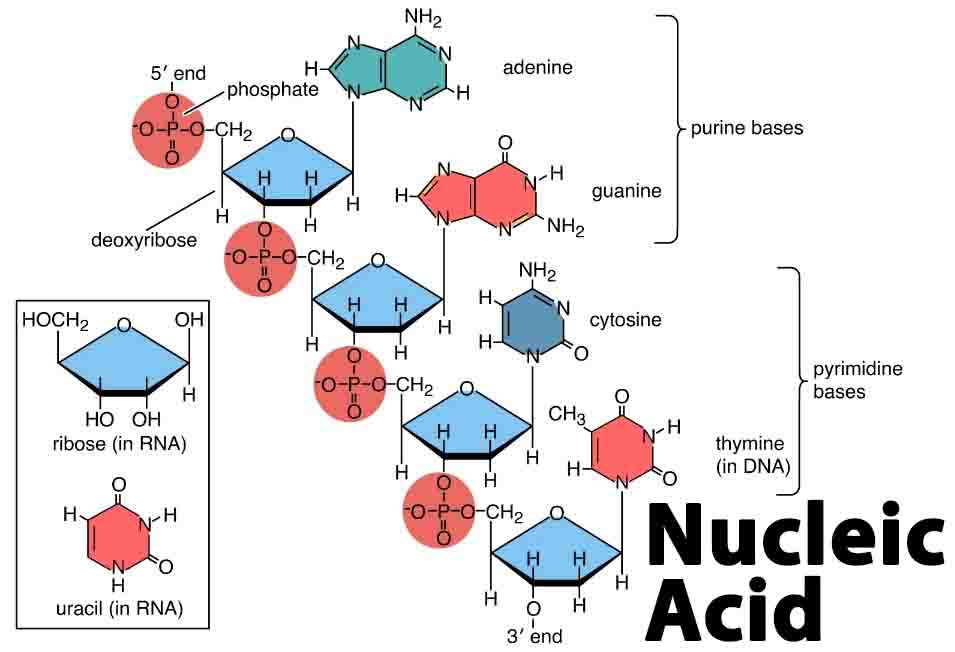
Nucleic acids are biocompounds, which are essential for living organisms. Found in two forms—deoxyribonucleic acid (DNA) and ribonucleic acid (RNA)—these polymer chains are composed of the same basic elements and similar monomer nucleotides, yet with specific differences relating to form and function.
Nucleic Acid Elements
Each nucleotide monomer, and therefore each nucleic acid polymer, is composed of a group of five elements. These elements bind to form monosaccharides, phosphate groups, and nucleobases, otherwise known as nitrogenous bases. In both RNA and DNA the phosphate group is the same form, but there are differences in the nitrogenous bases and sugar molecules.
The five elements necessary to construct a nucleic acid chain are carbon, hydrogen, oxygen, nitrogen, and phosphorus. The addition of phosphorus makes nucleic acid different to other categories of biocompounds, namely carbohydrates, lipids, and proteins.
Nucleic Acid Monomers
The chemical formulas of nucleic acid monomer show the quantities of each element. Nucleotide monomers are named according to the type of nitrogenous base they contain. When free, these monomers may have extra phosphate groups and be found in diphosphate, triphosphate, or polyphosphate forms.
Upon the formation of an RNA or DNA polymer, additional phosphate groups are released, leaving just one attached to the monosaccharide. The combination of ribose or deoxyribose and phosphate group forms the sugar-phosphate backbone. The nitrogenous base is attached to the sugar molecule.
The addition of a phosphate group to the nucleoside created by sugar and nitrogenous base forms a nucleotide. The nucleotide monomer therefore has various specifically-named structures—the sugar-phosphate backbone, the nucleoside, and the singular molecules of nitrogenous base, pentose sugar, and phosphate group.
In nucleic acids, pentose sugars come in two different forms, ribose and deoxyribose. The former possesses an additional oxygen molecule, which, in combination with hydrogen, forms a hydroxyl group. This feature is absent in deoxyribose.
Nitrogenous bases are categorised according to size. Double-ringed forms, called purines, are larger and longer and contain five nitrogen atoms. Single ringed forms, known as pyrimidines, contain between two and three nitrogen atoms and are smaller and shorter.
This is important in the double-strand feature of DNA and the process of translation, as only certain pairings of nitrogenous bases are possible (Watson-Crick pairings). These keep two strands equidistant from each other.
A mnemonic to assist with remembering which nucleotides belong to which group is the phrase ‘Pure As Gold’; it goes without saying that the remaining bases belong to the pyrimidine group. This also tells us that adenine and guanine cannot create a double-strand bond together. In RNA, other base combinations are possible and are known as non-Watson-Crick pairings.
In Watson-Crick pairings, larger bases, adenine, and guanine will never pair with each other. Similarly, purines do not connect to each other (cytosine, thymine, and uracil). In DNA, adenine only pairs with thymine and guanine with cytosine. In RNA, adenine pairs with uracil and guanine with cytosine.
The following images show the chemical structure of each type of monomer, where the pentagonal shape of the monosaccharide and its attached phosphate group and specific nucleobase are clearly defined.
Adenosine Monophosphate (AMP): C10H14N5O7P
This chemical formula represents the sum of the purine base adenine (C5H5N5), ribose (C5H10O5), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). This is the RNA form.
Deoxyadenosine Monophosphate (dAMP): C10H14N5O6P
This chemical formula represents the sum of the purine base adenine (C5H5N5), deoxyribose(C5H10O4), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). This is the DNA form.
Guanosine Monophosphate (GMP): C10H14N5O8P
The sum of the purine base guanine (C5H5N5O), ribose (C5H10O5), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). This is the RNA form.
Deoxyguanosine Monophosphate (dGMP): C10H14N5O7P
The sum of the purine base guanine (C5H5N5O), deoxyribose (C5H10O4), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). This is the DNA form.
Uridine Monophosphate (UMP): C9H13N2O9P
The sum of the pyrimidine base uracil (C4H4N2O2), ribose (C5H10O5), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). Only found in RNA.
Cytidine Monophosphate (CMP): C9H14N3O8P
The sum of the pyrimidine base cytosine (C4H5N3O), ribose (C5H10O5), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). This is the RNA form.
Deoxycytidine Monophosphate (dCMP): C9H14N3O8P
The sum of the pyrimidine base cytosine (C4H5N3O), deoxyribose (C5H10O4), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). This is the DNA form.
Thymidine Monophosphate (TMP): C10H15N2O8P
The sum of the pyrimidine base thymine (C5H6N2O2), deoxyribose (C5H10O4), and phosphoric acid (H3PO4), where condensation reactions at the molecule bond sites lose two water molecules (2H20). Only found in DNA.